Learn about the NIH and NIAMS policies and requirements for Data and Safety Monitoring (DSM), guidelines for creating a Data and Safety Monitoring Plan (DSMP), risk assessment and determination of monitoring type, types of monitoring bodies the NIAMS may consider, and much more.
Additional reporting requirements, such as the Institutional Review Board (IRB), the Office for Human Research Protections (OHRP) and/or the U.S. Food and Drug Administration (FDA), are separate from these guidelines and are the responsibility of the Principal Investigator and/or the institution receiving NIH funding, as appropriate.
TABLE OF CONTENTS
- 2.1 Scope
- 2.2 Data and Safety Monitoring Overview
- 2.3 Different Types of Monitoring Bodies that the NIAMS May Consider
3.0 Information for Applicants
5.0 The Data Safety Monitoring Plan Overview
6.0 NIAMS Risk Assessment and Determination of Monitoring Type
- 6.1 Data and Safety Monitoring Board (DSMB)/Observational Study Monitoring Board (OSMB)
- 6.2 Safety Officer/Dual Safety Officer
- 6.3 Internally-appointed Monitoring
1.0 NIH Policy
It is the policy of the National Institute of Health (NIH) that a system be in place for appropriate oversight and monitoring to ensure the safety of participants and the validity of data in all NIH-supported or conducted clinical trials. The NIH Policy for Data and Safety Monitoring (released on June 10, 1998) provides each Institute and Center (IC) with the flexibility to implement the requirement for data and safety monitoring as appropriate for its clinical research activities. Further guidance (NIH) related to the policy released in June 2000 stated that investigators must submit a detailed monitoring plan for review and approval by the IC for clinical trials before the trial begins.
2.0 Background
The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) supports clinical research which includes interventional and non-interventional (e.g., observational) studies of varying size and complexity. In order to fulfill its mandate to ensure a monitoring system is in place, as required by NIH Policy, the NIAMS has established the following system for the appropriate oversight and monitoring of the conduct of NIAMS-funded clinical research. The goal is to ensure the safety of research participants, the appropriate and ethical conduct of the study, the reliability, validity and integrity of the data, and the availability of data and dissemination of research results in a timely manner. The NIAMS believe that effective oversight, coupled with appropriate stewardship of funds, will ensure high-quality research. These guidelines replace the NIAMS Policy for Data and Safety Monitoring of Clinical Research released on August 1, 2017.
2.1 Scope
The NIAMS DSM guidelines apply to all NIAMS-funded grants, cooperative agreements, or contracts that include clinical research. Different types of human subjects research studies are supported by these funding mechanisms including but not limited to epidemiological and behavioral studies, clinical trials, outcomes research and health services research. For the purposes of this document, an NIH-defined clinical trial is a research study in which one or more human subjects are prospectively assigned to one or more interventions (which may include placebo or other control) to evaluate the effects of those interventions on health-related biomedical or behavioral outcomes. These may include studies of drugs, biologics, devices, surgical treatments, or behavioral interventions. The NIAMS policy applies to trials of all phases (e.g., Phase I – Phase IV) and stages as depicted in Figure 1. For more information about clinical trials, see NIH's Definition of a Clinical Trial.
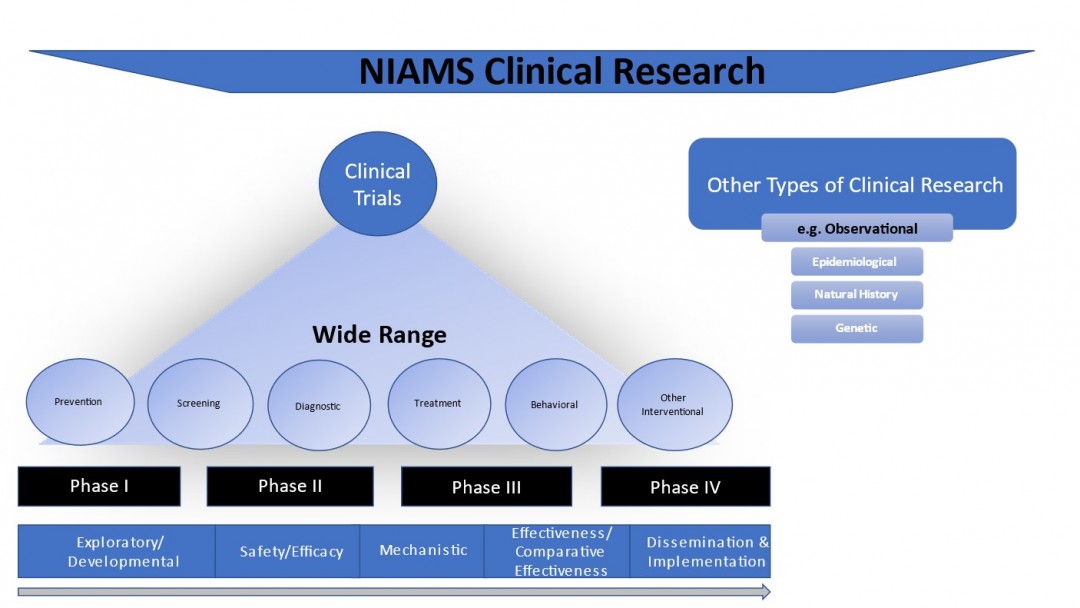
2.2 Data and Safety Monitoring Overview
The type of monitoring plan and DSM body may differ greatly between studies. All clinical research will be monitored, at minimum, by the Principal Investigator (PI) and Institutional Review Board (IRB), and some studies may require additional levels of data and safety oversight and monitoring. Clinical trials require additional monitoring by a committee, also known as a Data and Safety Monitoring Board (DSMB), or single or dual Safety Officer(s) (SOs). Large observational studies may require additional oversight by an Observational Study Monitoring Board (OSMB). Oversight by a DSMB, OSMB, or SO(s) could be either NIAMS-appointed or PI-appointed (referred to as internally-appointed monitoring). The NIAMS makes the final determination on the level of monitoring a clinical study will require.
The method or level of data and safety monitoring will be commensurate with the risks involved in the study (i.e., intervention risk or severity of the disease and risk to population being studied), the size and scope of the study, and the complexity of the trial (e.g., multi-site or NIH-defined Phase III trial). All human subjects research studies, including studies claiming human subjects exemption, require the submission of Section 3 - Protection and Monitoring Plans as part of the Public Health Service (PHS) Human Subjects and Clinical Trials Information Form (G.500). Section 3.1- Protection of Human Subjects requires non-exempt human subjects research studies to discuss risks of the study commensurate with its size and complexity. Applicants should provide a discussion of risks organized into four sections: Risk to Human Subjects; Adequacy of Protection Against Risks; Potential Benefits of the Proposed Research to Research Participants and Others; and Importance of the Knowledge to be Gained. Other information may be required as specified by the Funding Opportunity Announcement (FOA). Note: The NIAMS determination of risk for the purposes of determining the type of monitoring include risk to human subjects and the risk to the funding IC which may include financial and operational risk factors. The NIAMS has developed internal guidance to aid in determining the appropriate level of monitoring for a given study based on the different types of risks involved.
Additionally, all clinical trial applications submitted to the NIH must include a proposed Data and Safety Monitoring Plan (DSMP). This is another component of the PHS Human Subjects and Clinical Trial Information Form (G.500) found in Section 3.3 - Data and Safety Monitoring Plan. The DSMP developed by the PI for the application describes generally the overall framework for monitoring, including the process by which Adverse Events (AEs), Serious Adverse Events (SAEs), and Unanticipated Problems (UPs) will be managed and reported, as required by the IRB, the person or group responsible for monitoring, the awarding IC, the NIH Office of Biotechnology Activities, and the Food and Drug Administration (FDA) and the frequency of monitoring activities by various entities.
2.3 Different Types of Monitoring Bodies that the NIAMS May Consider
The NIAMS ultimately determines the appropriate type of DSM body required to conduct data and safety oversight for clinical studies it supports. A risk assessment is conducted for all clinical trials prior to award and allows NIAMS to make a determination for the type of DSM body the study must utilize for data and safety oversight. The following are different types of monitoring the NIAMS may consider.
2.3.1 NIAMS-appointed DSMB
A DSMB is an independent group of experts that advises the NIAMS on the conduct of clinical trials and must be free from conflicts with members of the study team. The DSMB is charged with reviewing study data for participant safety; study conduct and progress; and providing formal recommendations to the NIAMS regarding study continuation, modification, and/or termination. For the majority of studies that require a DSMB, NIAMS will select and appoint the board members. All NIH-defined Phase III trials require DSMB oversight. DSMB oversight may also be considered for pilot and feasibility trials and Phase I, II, and IV trials, dependent upon the potential risks, complexity, and size of the trials.
2.3.2 NIAMS-appointed OSMB
An OSMB is an independent group of experts that advises the NIAMS and must be free from conflicts with members of the study team. The main difference between an OSMB and a DSMB is that an OSMB is utilized for oversight and monitoring of observational studies (rather than clinical trials). Typically, observational studies are low-risk to participants, but may be large in size (i.e., number of sites or number of participants) which increases the complexity of the study and risk to data quality and integrity. These factors, along with other elements of the observational study may require it to have an OSMB. Similarly, to a DSMB, the OSMB is charged with reviewing study data, but participant safety may not be the main responsibility like it is for a DSMB. The OSMB monitors study conduct and progress, and provides formal recommendations to the NIAMS regarding study continuation, modification, and/or termination.
2.3.3 NIAMS-appointed Safety Officer (SO)/Dual SO
An SO is an independent expert, typically a physician (M.D.), and must be qualified to assess safety matters related to the population, disease, and intervention under study. The SO advises the NIAMS on the conduct of clinical research studies, including clinical trials, and must be free of conflicts from the study team. The SO is charged with monitoring study progress, data quality, and accumulating safety data, in order to alert the Institute regarding any potential safety or other monitoring concerns affecting the conduct of the study. An SO is typically used for studies that are small, short-term or low-moderate risk, and where a DSMB/OSMB oversight may not be necessary or appropriate. The SO reviews comprehensive safety reports prepared by the PI and provides a recommendation regarding the study, continuation, modification, and/or termination. For some studies, the NIAMS may determine the study requires monitoring by two experts, rather than one, which will result in monitoring by “Dual SOs”.
2.3.4 Internally-appointed monitoring
Internally-appointed monitoring is a term used by the NIAMS to describe data and safety monitoring oversight by a designated individual or group of individuals who are appointed by the PI (rather than the NIAMS) in consultation with the NIAMS, and assembled and managed by the PI and/or Institution. The internally-appointed DSM body can be either a DSMB/OSMB or SO and should remain independent (i.e., free of conflicts) of the investigator(s) participating in the study. Studies with this type of monitoring are determined to be low-risk by the NIAMS. The NIAMS is required to be kept informed of the study’s progress and outcome of the DSM body’s review of data following any meetings or review of data and safety reports.
3.0 Information for Applicants
The DSMP is a required attachment to Section G.500 - PHS Human Subjects and Clinical Trials Form and is submitted as part of the research application for all clinical trials applying for NIH Extramural funding. Instructions on what to include in the DSMP are listed in Section 3.3 - Data and Safety Monitoring Plan. This plan will be reviewed by a scientific review group (SRG) and is considered under the Additional Review Criteria/Human Subjects part of the application, and any comments and concerns will be notated by the Scientific Review Officer (SRO) in the summary statement under the Human Subjects section.
4.0 Information for Grantees
For NIAMS-funded studies, the Institute ultimately determines the appropriate type of monitoring for data and safety oversight (see Section 6 for NIAMS Risk Assessment Procedures and Determination of Monitoring type). The type of DSM body determined by the NIAMS may differ significantly from what was originally proposed in the DSMP submitted as part of the research application and may need to be revised to reflect any changes during the post-award review. The NIAMS provides a DSMP template to investigators to help with the development of a final, detailed DSMP. The DSMP will be reviewed by the NIAMS, NIAMS Executive Secretary (ES), and NIAMS-appointed DSM body (if applicable) prior to study initiation.
5.0 The Data and Safety Monitoring Plan Overview
5.1 What is it?
The DSMP serves as a guide to ensure the PI has given consideration to the various aspects of the study which can impact data and safety of participants. All clinical trials are required by NIH policy to submit a DSMP that is commensurate with the risk of the trial, its size, and its complexity. Though per NIH requirements a DSMP is optional for all other types of clinical research (e.g., observational), the NIAMS requires a DSMP for all clinical research studies if monitored by a DSM body. More information about what may need to be included in a DSMP for a clinical study is found in Section 5.2. The DSMP provides a general description of procedures for safety monitoring as well as the various entities responsible for trial monitoring and advising the appointed DSM body. There are a number of options for monitoring that are possible and depend upon potential risks, complexity, size, and the nature of the trial. The entities monitoring the study may include, but are not limited to, monitoring by the PI and the IRB, an individual, typically a physician (e.g., SO), or committee of experts (e.g. DSMB, OSMB). When preparing the DSMP, the PI, and the study team should consider: the study protocol (i.e., design and complexity of the study), phase, intervention(s), target population, participant safety and privacy, risks and benefits involved in the study, data integrity and confidentiality, study coordination, and how the team will address each of these elements. The DSMP will be revised with input from the NIAMS, NIAMS ES, and DSM body once a study has been awarded. Please note that when proposing a DSMB/OSMB, only the general composition of the Board without naming specific individuals is advised to be included in the DSMP submitted with the grant application.
The DSMP should specify how data are to be presented and triggers for presenting safety data in an unmasked manner. In addition, the DSMP should specify the process for reporting safety concerns among the IRB, the SO/dual SO, the NIAMS and, if appropriate, the FDA and OHRP.
5.2 What should be included?
The NIAMS has developed a template to assist grantees with the writing of a DSMP for clinical trials. While this is normally used post-award to further develop or refine the DSMP submitted as an attachment to section 3.3, it is a helpful resource that applicants may also refer to when writing their DSMP for submission with the grant. See How to Write a Data and Safety Monitoring Plan to learn more. In general, the plan should include the following elements:
- Study Overview
- Study Description
- Study Management
- Participant Safety
- Potential Risks and Benefits for Participants
- Potential Risks
- Potential Benefits
- Protection Against Study Risks
- Informed Consent Process
- Potential Risks and Benefits for Participants
- Reportable Events
- Definitions
- Adverse Events (AEs)
- Serious Adverse Events (SAEs)
- Unanticipated Problems (UPs)
- Protocol Deviations
- Collection and Assessment of AEs, SAEs, UPs, and Protocol Deviations
- Reporting of AEs, SAEs, UPs, and Protocol Deviations
- AE Reporting Procedures
- SAE Reporting Procedures
- UP Reporting Procedures
- Protocol Deviations Reporting Procedures
- Serious or Continuing Noncompliance
- Suspension or Termination of IRB Approval
- Definitions
- Interim Analysis and Stopping Rules
- Data and Safety Monitoring
- Frequency of Data and Safety Monitoring
- Content of Data and Safety Monitoring Report
- Monitoring Body Membership and Affiliation
- Conflict of Interest for Monitoring Bodies
- Protection of Confidentiality
- Monitoring Body Responsibilities
- Data Management, Quality Control, and Quality Assurance
Unlike a DSMP for a clinical trial, a DSMP for other clinical research studies may not need to address all of the elements listed above. Generally, the NIAMS requires a DSMP for these clinical research studies to include, but not be limited to the following elements:
- Study Overview
- Participant Safety
- Reportable Events
- Collection and Assessment of AEs, SAEs, UPs, and Protocol Deviations
- Reporting of AEs, SAEs, UPs, Protocol Deviations, Serious or Continuing Noncompliance and Suspension or Termination of IRB Approval
6.0 NIAMS Risk Assessment and Determination of Monitoring Type
Once a clinical trial has been approved for funding, the NIAMS uses its internal process to conduct a risk assessment using a risk and safety assessment tool. This tool is used to determine the level of risk of a clinical trial, taking into consideration the study type, phase, intervention, population, research environment, risk to human participants and the adequacy of human subjects protections. Additional factors that the NIAMS considers when determining risk include, but are not limited to:
- Funding mechanism and complexity of grant
- Budget considerations
- Operational and Institutional risk to the NIAMS
- Prior experience, training and history of PI conducting clinical trials
A detailed review of information pertaining to the various risks are considered and the NIAMS makes a determination for the type of safety monitoring it will require for data and safety oversight for the study.
6.1 Data and Safety Monitoring Board (DSMB)/Observational Study Monitoring Board (OSMB)
The NIH policy for data and safety monitoring specifically requires the establishment of DSMBs for multi-site clinical trials involving interventions that pose potential risk to participants. Interventional studies that are high-risk (regardless of whether they are single or multi-site) and NIH defined Phase III clinical trials require monitoring by a NIAMS-appointed DSMB.
Non-interventional clinical research studies that are generally low risk, and either single-site or multi-site, may require monitoring by a NIAMS-appointed OSMB. Please note that the NIAMS’ determination for a NIAMS-appointed DSMB/OSMB includes many factors such as, but not limited to; number of sites, risk to human subjects, number of participants, phase of study, type of population, study complexity, and type of intervention. The NIAMS also takes into consideration other factors mentioned in Section 6. NIAMS Risk Assessment and Determination of Monitoring Type.
6.1.1 Composition
Appointment of the DSMB/OSMB members is the responsibility of the NIAMS. However, the study investigators may make suggestions to the NIAMS for consideration of potential candidates to serve on the DSMB/OSMB. The NIAMS makes the final decision on the selection of the DSM body candidates.
All DSMB/OSMB members have voting privileges and are selected for their appropriate expertise within the relevant scientific and methodological areas needed to objectively oversee a study and may include, but are not limited to; clinical trialists, clinicians, laboratory scientists, statisticians, and ethicists. The NIAMS prefers one statistician and one bioethicist to be members of every DSMB/OSMB. DSMBs/OSMBs can range in size from 3-7 members, dependent upon the monitoring and oversight needs of the study. The NIAMS determines the expertise and number of members required for a DSMB/OSMB.
Membership should consist of persons completely independent of the investigators who have no financial, scientific, or other relevant conflicts of interest with the study/trial. Current or past (within the last three years) collaborators or associates of the PI or any co-investigators, such as those in the same institution, are not eligible to serve on the DSMB/OSMB. The NIAMS carefully reviews all potential members’ disclosures carefully prior to making a selection for the Board. Conflicts are checked annually, at a minimum, and members are required to disclose new information as it arises and attest to the absence of conflicts in writing.
Individuals with additional expertise may be invited by the NIAMS to participate as ad-hoc members in the DSMB/OSMB meeting or to advise on emerging issues, as deemed necessary by the NIAMS. Ad-hoc members must also be independent of the study/trial and have no financial, scientific, or other relevant conflicts of interest with the PI or any co-investigators. Written documentation attesting to the absence of conflicts of interest is required before any member starts his/her participation.
The DSMB/OSMB Chairperson is appointed by the NIAMS and confirmed by a full DSMB/OSMB vote at the first meeting. The DSMB/OSMB Chairperson is typically a person with past experience serving on DSMBs/OSMBs and may be a clinical expert, although it is not required. The Chairperson is responsible for leading the meetings, working with the NIAMS and/or the NIAMS ES to develop the agenda, and summarizing all DSMB/OSMB recommendations to the DSMB/OSMB, the NIAMS, and the NIAMS ES, with input from the DSMB/OSMB in the Executive Session. The Chairperson is the primary contact person for the DSMB/OSMB. The NIAMS consults with the DSMB/OSMB Chairperson on administrative and logistical issues related to the meetings as they arise.
One, and sometimes two, member(s) will be selected as the DSMB/OSMB SO(s) and will be the primary contact person(s) for independent review and assessment of expedited safety reports (e.g., SAEs, protocol deviations, and unanticipated problems) submitted by the PI and study team. The SO(s) are also appointed by the NIAMS and confirmed by a full DSMB/OSMB vote at the first DSMB/OSMB meeting. SO(s) must possess relevant medical expertise to provide input on safety related matters related to the disease, population, and intervention under study.
NIAMS staff are not members of the DSMB/OSMB, and do not have voting privileges. The NIAMS should not participate in discussion during the DMSB meeting unless it is to raise questions to seek clarification from the DSMB/OSMB and the investigators or to offer information regarding a NIH/NIAMS policy or to clarify a meeting process. The NIAMS may not influence or provide input into the DSMB/OSMB recommendations, so that the DSMB/OSMB can maintain its objectivity and independence.
6.1.2 PI Expectations and Guidelines for Reporting to a DSMB/OSMB (PI Responsibilities)
The role of the clinical study PI is to ensure the study is conducted following the protocol and according to the highest scientific and ethical standards. Outlined below are the PI responsibilities as it relates to data and safety monitoring.
6.1.2.a Prior to the First DSMB/OSMB Meeting
Suggest candidates to the NIAMS Program Director to serve as members of the DSMB/OSMB – Potential candidates should possess appropriate expertise in the relevant scientific and methodological areas and may include clinicians, statisticians, and ethicists. Members must be completely independent of the investigators and have no financial, scientific, or other conflicts of interest with the study.
- Submit a revised DSMP to the NIAMS for review and approval, at least 4-6 weeks prior to the meeting. Revise DSMP, as requested by the NIAMS.
- Submit the study materials including a study protocol, Manual of Operating Procedures (MOP), and the informed consent/assent form(s) to the NIAMS for review and approval, at least 4-6 weeks prior to the meeting. Revise study documents, as requested by the NIAMS.
- Submit FDA approval(s) or relevant correspondence regarding IND/IDE, if applicable, and IRB approval letter(s) to the NIAMS for record.
- In consultation with the study statistician, draft the routine DSM report templates to be used to present study data at DSMB/OSMB meetings – The NIAMS DSM Report Templates may be used and modified, as needed. Submit to the NIAMS, at least 4-6 weeks prior to the meeting. Reports should include a brief narrative description of the study background, status, significant problems, and proposed resolutions. The narrative is followed by a series of tables. These reports must be customized to meet the needs of each particular study.
- Prepare a presentation about the study protocol to give at the first DSMB/OSMB meeting. Submit to the NIAMS 1-2 weeks prior to the meeting. A PowerPoint presentation approximately 20 to 30 minutes in length should be prepared. The presentation should include study objectives and design, plans for recruitment, safety monitoring plan which include report templates (can be presented by the study statistician or data manager), determination and reporting of AEs, data management and analysis plan and the study status and timeline. The presentation should touch on significant areas of the protocol but not repeat the entire protocol.
- Review the DSMB/OSMB Charter drafted by the NIAMS. The PI may ask questions and request clarification at the meeting.
6.1.2.b The First DSMB/OSMB Meeting
The NIAMS determine the timeline for the initial DSMB/OSMB meeting. The first meeting is held either by web-conference or in-person prior to the study initiation and lasts approximately three to four hours.
Attendees include: the PI, co-investigators, study statistician, other essential/key study team members (e.g., Clinical Research Coordinator, Project Manager), DSMB/OSMB members, the NIAMS staff, and the NIAMS ES. The PI/study team should not extend the meeting invitation to others to join the meeting unless it has been approved by the NIAMS.
The NIAMS ES solicits availabilities, schedules and facilitates the meeting, and reviews and distributes study materials that are prepared by the study personnel to the DSMB/OSMB members and NIAMS staff. If there is an in-person meeting, travel arrangements for the study team attendees will be the responsibility of the study team, per the grant. Travel arrangements for the DSMB/OSMB members are arranged by the DSMB/OSMB members, but members are reimbursed by the NIAMS. At the first meeting:
- The NIAMS provides a training presentation to explain the roles and responsibilities of the NIAMS, DSMB/OSMB, and PI regarding the data and safety monitoring process.
- The DSMB/OSMB members vote to confirm the NIAMS nominated Chairperson and SO.
- The DSMB/OSMB members vote to approve the DSMB/OSMB Charter.
- The PI gives a presentation about the study protocol and answers questions from the DSMB/OSMB members regarding the study and the study materials reviewed.
- The study statistician/data manager presents report templates that will be used to report study data at future meetings.
- DSMB/OSMB members discuss and decide on the content of DSMB/OSMB reports for future meetings.
- The DSMB/OSMB deliberates on any issues raised during the discussions and formulates recommendations for the NIAMS review and acceptance.
- The DSMB/OSMB may vote for the study to begin enrollment, contingent upon satisfactory responses to the recommendations, or may postpone that vote to a later time.
6.1.2.c Subsequent DSMB/OSMB Meetings
The NIAMS ES schedules all meetings. Subsequent meetings are usually held semi-annually, either via web-conference (approximately 2 hours) or in-person (approximately 3 hours). Additional meetings may be held ad-hoc or routinely, if deemed necessary.
- The PI/study statistician provides the NIAMS ES with the DSMB/OSMB report at least 2 weeks before a meeting. Once a study begins, the study data coordinating center, statistical office or statistician will prepare reports as agreed upon with the DSMB/OSMB during the first DSMB/OSMB meeting. The reports should be clearly labeled to identify open and closed sessions, as relevant.
- The PI presents an update on the progress of the study and any protocol changes or issues that require the input of the DSMB/OSMB.
- The study statistician presents aggregate safety data and progress.
- If a closed session is required, the unblinded statistician presents unmasked safety data.
- The DSMB/OSMB discusses and deliberates on the information presented by the PI/study team and formulates recommendations for the NIAMS review and acceptance.
- The DSMB/OSMB votes for the study to continue or to be stopped either temporarily or permanently based on accumulating data and information.
6.1.2.d General Format of DSMB/OSMB Meetings
General (Open) Session
- Includes the PI and study team, DSMB/OSMB members, the NIAMS, and the NIAMS ES.
- An annual Charter review is conducted.
- Meeting minutes from the prior meeting are reviewed and voted on by DSMB/OSMB members for acceptance and official documentation for the meeting record.
- The PI response to recommendations from prior meeting is reviewed by DSMB/OSMB members for acceptance and official documentation for the meeting record.
- The PI presents the study’s key points, progress, and any issues/new information to the DSMB/OSMB.
- The open session DSMB/OSMB report is presented including enrollment, safety and data quality tables and listings.
- Discussion and selection of timing of next meeting.
Closed Session (if needed)
- Includes the DSMB/OSMB, the unblinded study statistician, the NIAMS, and the NIAMS ES.
- Safety data is presented by unmasked treatment group (e.g., placebo vs. steroid) or masked treatment group (e.g., A vs. B) by the study statistician.
- Outcome data is also presented, if it is requested by the DSMB/OSMB members.
- Interim analysis may be presented, if required.
Executive Session
- Includes the DSMB/OSMB members, the NIAMS, and the NIAMS ES.
- The DSMB/OSMB discusses study status, data presented, protocol changes, and deliberates to formulate recommendations for the NIAMS.
- Vote on study status (start, stop, put on hold).
6.1.2.e Follow-up After a DSMB/OSMB Meeting
- Meeting Recommendations:
- The NIAMS ES transcribes the meeting recommendations within two business days after the meeting and circulates them to the DSMB/OSMB for review. Once the DSMB/OSMB has provided its review and any editorial changes/clarifications (typically requested within two business days), the recommendations are sent to the NIAMS for review and acceptance.
Note: The NIAMS may not accept all recommendations and will amend any recommendations it does not accept. The amended recommendations are re-circulated to the DSMB/OSMB for their information. Recommendations are final and the PI must respond to all with either an acknowledgement or explanation of how the recommendation will be implemented. If the PI disagrees to implement a recommendation, a justification must be provided to the NIAMS and the DSMB/OSMB through the NIAMS ES. The NIAMS will consider if it accepts the justification, and if other action must be taken.
- The NIAMS ES transcribes the meeting recommendations within two business days after the meeting and circulates them to the DSMB/OSMB for review. Once the DSMB/OSMB has provided its review and any editorial changes/clarifications (typically requested within two business days), the recommendations are sent to the NIAMS for review and acceptance.
- The NIAMS ES will only distribute the final recommendations to the PI, study team members, and all other participants who attended the open session. It is the responsibility of the PI to share the recommendations with other members of the study.
Note: If there were recommendations pertaining to a closed discussion, those recommendations are shared separately only with the study statistician who attended the closed session of the meeting. - The PI submits a written response to the meeting recommendations within a specified timeframe (typically 30 days). Some recommendations will require an acknowledgment, and no action by the PI. Others will require a response to explain how the recommendation(s) will be implemented.
- The NIAMS ES transcribes the meeting minutes within five business days after the meeting and circulates them to the DSMB/OSMB for review. Once the DSMB/OSMB has provided its review and any editorial changes/clarifications (typically requested within three business days), the minutes are sent to the NIAMS for review and any editorial changes/clarifications.
- The minutes are finalized by the NIAMS ES and the open session minutes are sent to all meeting participants who attended the open session of the meeting. The closed and executive sessions meeting minutes are separate and shared only with the participants who attended those sessions.
6.1.3 Roles and Responsibilities of the DSMB/OSMB
The DSMB/OSMB acts in an advisory capacity to the NIAMS as an independent body. The DSMB/OSMB is responsible for reviewing accumulating safety data and study/trial progress to assure that the study participants are not exposed to unnecessary or unreasonable risks, and the investigator conducts the study/trial according to the protocol and highest scientific and ethical standards.
DSMB/OSMB members are paid an honorarium by the NIAMS for their participation in DSMB/OSMB meetings, which includes contributing their time and efforts to the review of the study materials and the ongoing review of safety reports, as well as other requests as needed.
Once a study/trial is implemented, ongoing responsibilities of the DSMB/OSMB are to:
- Review the study protocol, DSMP, MOP, and informed consent documents, including all proposed revisions.
- Evaluate the progress of the study on an ongoing basis, including periodic assessments of data quality, participant recruitment, accrual and retention, participant risk versus benefit, performance of study site(s), and other factors that can affect the outcome.
- Evaluate safety throughout the course of the study through the routine review of aggregated AE safety data, in addition to expedited review of UPs, SAE reports, and protocol deviations impacting participant safety. The DSMB/OSMB SO reviews the documentation provided by the study team and makes recommendations to the NIAMS regarding protection of the study participants.
- Evaluate proposals of new sites (that differ from the approved application) and make a recommendation to the NIAMS as to whether the enrollment at the site(s) is expected to enhance overall enrollment. Activities include evaluating the patient population pool, catchment area description, recruitment plan, and target enrollment for any new clinical sites.
- Consider the impact of factors external to the study when new information, such as scientific or therapeutic developments, becomes available and may affect safety of participants, their willingness to participate in the study or the ethics and conduct of the study.
- Assist the NIAMS by commenting on any problems with study conduct or performance.
- Ensure that the plan for maintaining the confidentiality of the study data and the results by the study team is appropriate.
- Review and evaluate requests for protocol modifications.
- Review in advance of the study initiation the study specific stopping rules and plans for interim analyses as established by the PI and selected members of the study team. These plans outline the conditions under which a study may be stopped (e.g., difficulties in recruitment, retention, obtaining outcome measures, or other issues).
- Review the interim analyses and/or accumulating data at the specified interval(s), and as appropriate and make a recommendation to continue, terminate, or modify the study based on observed benefit or harm in accordance with the planned stopping rules.
6.1.3.a Study Materials
Study materials (e.g., protocol, MOP, DSMP, informed consent/assent form(s), DSM report templates, case report forms (CRFs), and any other materials required for the DSMB/OSMB’s review) are prepared by the PI and the study team. The DSMB/OSMB must initially review the materials prior to the commencement of recruitment to ensure they are adequate to implement the study. During the introductory meeting, the DSMB/OSMB will provide comments to clarify or revise parts of the study materials, if required. They will make a recommendation regarding the adequacy of the study materials prior to the start of the study.
As the study progresses, it is expected that there may be unanticipated changes to the study protocol that require updates to the study materials. Any changes to the study potentially impacting the budget must first be discussed with the NIAMS program staff. Amended materials include but are not limited to changes to the protocol, MOP, DSMP, informed consent/assent form(s), and CRFs. All changes made to the study should be reviewed by the DSMB/OSMB, the NIAMS, and the IRB prior to implementing the changes.
6.1.3.b DSMB/OSMB Charter
The purpose of the Charter is to outline the charge to the DSMB/OSMB regarding its responsibilities, composition, and processes for a NIAMS-funded study. It also outlines the responsibilities of the NIAMS and the NIAMS ES. It is intended to be a living document to be modified at any time if any processes or procedures were to change. The Charter is created by the NIAMS for individual studies that require NIAMS-appointed DSMB/OSMB oversight. The NIAMS ES helps facilitate modifications to the Charter.
A formal review and full DSMB/OSMB vote to approve the Charter take place at the introductory meeting, and a re-review occurs on an annual basis or as needed. The DSMB/OSMB may request modifications to the Charter to align with any specific charge it has to the study. The Charter template can be accessed on the NIAMS website at https://www.niams.nih.gov/sites/default/files/DSMB-Charter-Template-2020-2.pdf.
6.1.3.c Confidentiality
All materials, discussions, and proceedings of the DSMB/OSMB are completely confidential. Each DSMB/OSMB member signs a confidentiality form attesting to adhering to confidentiality. This process is completed annually by each DSMB/OSMB member. Study team members and other participants in the DSMB/OSMB meetings are also expected to maintain confidentiality.
6.1.3.d DSM Reports
The PI is required to routinely report on the status of ongoing study activities with emphasis on data integrity and participant safety issues for the DSMB/OSMB’s review. If the NIAMS DSM Report templates are used, they must be customized to each study and the DSMB/OSMB may request additional reports, as necessary. View the NIAMS DSM Report Template single-site open session, single-site closed session, multi-site open session, and multi-site closed session. The PI can create his/her safety reports, however, they must be reviewed and approved by the DSMB/OSMB. The DSMB/OSMB has the ability to review and may request unmasked clinical trial data by group (e.g., placebo vs. steroid) at its discretion. The DSMB/OSMB must decide the format in which it will review closed session data (i.e., masked vs. unmasked) at the initial meeting, if applicable.
6.1.3.e AE, SAE, UP, and Protocol Deviation Definitions
The PI and study team are expected to provide a definition of AEs, SAEs, UPs, and protocol deviations. The PI and study team may draw from OHRP guidance (https://www.hhs.gov/ohrp/regulations-and-policy/guidance/reviewing-unanticipated-problems/); for some studies, the ICH E6 definition may be more appropriate.
Define the events placing the participant at increased risk of harm or compromise the integrity of the safety data for all protocol deviations.
6.1.3.f AE Reporting
In the case where the intervention is an FDA regulated drug, device or biologic, it should include the FDA definition, grading scale and “study relatedness” criteria of AEs. All non-serious AEs (regardless of expectedness or relatedness) are reported to the DSMB/OSMB and the NIAMS (through the NIAMS ES) semi-annually as part of the routine DSM report, or as determined by the NIAMS. For more information, see the NIAMS Reportable Events Requirements and Guidelines for Investigators Conducting NIAMS-Funded Clinical Research Studies.
6.1.3.g Expedited Reporting of SAEs, UPs, and Protocol Deviations
The DSMB/OSMB SO is the designated DSMB/OSMB member responsible for reviewing all SAEs and other expedited reports (e.g., UPs, protocol deviations impacting the safety of the research participants) as soon as they are reported by the investigator. Expedited reports are submitted to the NIAMS and the DSMB/OSMB SO through the NIAMS ES within a predetermined interval (within 48 hours of the investigator becoming aware of the event). If a full report cannot be submitted within 48-hours, an initial SAE report may be submitted followed by a full report. The NIAMS and DSMB/OSMB SO will review the information simultaneously provided by the investigator, typically contained in an SAE form, and independently assess the relationship of events to study/intervention. Additional information may also be requested by the NIAMS and DSMB/OSMB SO to aid in their review of the event. In case the NIAMS and DSMB/OSMB SO agrees with the investigator assessment, the results will be shared with the investigator, and no changes to the SAE report will be required.
In the event the NIAMS and the DSMB/OSMB SO disagrees with the investigator’s assessment of the event, the difference in adjudication will be shared with the investigator for comment and to provide a rationale for his/her assessment. If the DSMB/OSMB SO and investigator cannot come to a mutual agreement, the DSMB/OSMB Chairperson may be requested by the NIAMS to weigh in on the SAE report and the investigator and DSMB/OSMB SO assessments and provide his/her feedback. If for any reason a mutually agreed upon assessment still cannot be reached, the NIAMS will further discuss the appropriate action that should be taken to resolve the difference in assessment between the parties. If a resolution cannot be accomplished over email, an ad-hoc meeting will be coordinated between the parties, including but not limited to the investigator, DSMB/OSMB SO, DSMB/OSMB Chairperson (if applicable), and NIAMS. The investigator may be required to report the difference in AE assessment to the IRB. The NIAMS decision should be considered official and final, and further discussion with the DSMB/OSMB will not be needed.
The designated DSMB/OSMB SO is required to notify the NIAMS if a pattern of events occurs and will suggest prevention measures (e.g., modifying the protocol to require frequent measurement of laboratory values predictive of the event). All SAEs reported expeditiously will be reviewed by the full DSMB/OSMB during the semi-annual meetings. It will provide an opportunity for the other members to review all safety data.
The DSMB/OSMB SO may request individual patient records, including laboratory data, clinical records, and other study related data, to evaluate these events against the known safety profile of the study intervention and the disease. The DSMB/OSMB SO may recommend actions including partial or complete unblinding, and/or modifying or terminating the study.
6.1.3.h Interim Analysis Reports
Interim analysis may be conducted either due to pre-specified stopping rules as outlined in the protocol and at predetermined intervals, or as deemed necessary by the DSMB/OSMB to assess safety concerns or study futility, based upon accumulating data. An interim analysis may be performed for safety, efficacy and/or futility, and the reports are prepared by the unmasked study statistician or data coordinating center responsible for generating such reports. Interim analysis reports are presented during the closed session to the DSMB/OSMB in an unmasked format, without the masked study personnel in attendance.
- Interim analysis for safety: The DSMB/OSMB may review unmasked interim safety data and recommend termination or modification of a trial due to a trend toward showing harm to the research participants.
- Interim analysis for efficacy: The DSMB/OSMB may review unmasked interim efficacy data and recommend termination of the trial, based on the interim data, where there is overwhelming evidence that the intervention is highly beneficial.
- Interim analysis for futility: The DSMB/OSMB may review unmasked interim efficacy data and recommend termination of the trial with the likelihood that the treatment effect being sought, based on the interim data, is very unlikely to be established even if fully enrolled.
6.2 Safety Officer/Dual Safety Officer
Non-interventional clinical research studies that are generally low to moderate risk, and single-site, may require monitoring by a NIAMS-appointed SO or dual SO. Additionally, some clinical trials that are generally moderate to high risk, and single-site or multi-site, may also require safety monitoring by a NIAMS-appointed SO or dual SO. Please note that the NIAMS’ determination for a NIAMS-appointed SO(s) includes many factors such as, but not limited to: number of sites, risk to human subjects, number of participants, phase of study, study population, study complexity, and type of intervention. The NIAMS also takes into consideration other factors mentioned in Section 6.
While the SO is appointed by the NIAMS, the PI and study team members may provide suggestions to the NIAMS for consideration, of individuals who are qualified to serve in this capacity. The SO will usually be independent of the institution(s) and investigator(s) participating in the trial and should have relevant expertise in clinical trials and the disease area being studied. The SO must have no financial, scientific, or other relevant conflicts of interest. Like the DSMB/OSMB, the NIAMS makes the final decision on the selection of the SO candidate(s). The SO will be paid an honorarium by the NIAMS for contributing their time and efforts to the review of the study materials and semi-annual safety reports.
6.2.1 Guidelines for Reporting to an SO/Dual SO (PI Responsibilities)
The role of the PI is to ensure the study is conducted following the protocol and according to the highest scientific and ethical standards. Outlined below are the PI responsibilities as it relates to data and safety monitoring. Please note that unlike studies with DSMB/OSMB monitoring oversight, SO studies do not hold routine meetings between the NIAMS, PI and SO(s). Only an initial introductory meeting is held prior to the start of the study. No other meetings are conducted except in cases where an issue arises and the NIAMS or SO request an ad-hoc call. All data and safety monitoring are conducted electronically through the review of DSM reports.
The PIs responsibilities are outlined below:
6.2.1.a Prior to the First SO/Dual SO Meeting
- Suggest candidates to the NIAMS Program Director to serve as the SO/Dual SOs. Potential candidates should possess appropriate expertise in the relevant scientific and methodological areas, should be qualified to assess safety matters related to the population, disease, and intervention under study, and are preferably physicians (M.D.). Members must be completely independent of the investigators and have no financial, scientific, or other conflicts of interest with the study.
- Submit a revised DSMP to the NIAMS for review and approval, at least 4-6 weeks prior to the meeting. Revise DSMP, as requested by the NIAMS.
- Submit the study materials including a study protocol, MOP, and the informed consent/assent form(s) to the NIAMS for review and approval, at least 4-6 weeks prior to the meeting. Revise study documents, as requested by the NIAMS.
- Submit FDA approval(s) or relevant correspondence regarding IND/IDE, if applicable, and IRB approval letter(s) to the NIAMS for record.
- In consultation with the study statistician, draft the routine DSM reports to be used to submit study data to the SO/Dual SO electronically – Reports should include a brief narrative description of the study background, status, significant problems, and proposed resolutions. The narrative is followed by a series of tables. These reports must be customized to meet the needs of each study.
- Prepare a presentation about the study protocol to give at the first SO/Dual SO meeting – A presentation, using slides or other materials and limited to no more than 15 to 20 minutes in length, should be prepared and presented at the first meeting. The presentation should include study objectives and design, plans for recruitment, safety monitoring plan determination and reporting of AEs, data management and analysis plan and the study status and timeline. Presentation materials should be sent to the NIAMS ES at least two weeks prior to the meeting for immediate posting to the website. The presentation should touch on significant areas of the protocol but not repeat the entire protocol.
- Review the SO/Dual SO Charter drafted by the NIAMS – The SO/dual SO Charter outlines the charge to the SO/dual SO and is dynamic throughout the study. The SO/dual SO will provide suggestions for amending the Charter at the first meeting and/or vote for its approval.
6.2.1.b The First SO/Dual SO Meeting
The NIAMS determine the timeline for the initial SO/dual SO meeting. The first meeting is held by web-conference prior to the study initiation and lasts approximately two hours. Attendees include: the PI, co-investigator(s), study statistician, other essential/key study team members (e.g., Clinical Research Coordinator, Project Manager), the SO/dual SO, the NIAMS staff, and the NIAMS ES. The PI/study team should not extend the meeting invitation to others to join the meeting unless it has been approved by the NIAMS. At the meeting:
- The NIAMS provides a training presentation to explain the roles and responsibilities of the NIAMS, the SO/dual SO, and PI regarding the data and safety monitoring process.
- The PI gives a presentation about the study protocol – The purpose of this presentation is to provide the SO/dual SO with background about the study and explain the basics of the study protocol. The presentation should be limited to about 15 to 20 minutes.
- The SO/dual SO decides on the content of reports – The SO/dual SO reviews the DSM reports proposed by the study statistician and PI and provides instructions on what information they would like to be included in future reports. Requirements for an interim analysis, if applicable, will also be determined by the SO/dual SO at this meeting. Reporting requirements may be augmented as the study progresses.
- The SO may recommend that the study begin or may postpone that decision to a later time once some study changes are implemented.
6.2.1.c Subsequent Reporting
Subsequent reports are usually reviewed electronically on a semi-annual basis. Additional meetings may be held ad-hoc or routinely, if necessary.
Once a study begins, the study coordinating center or statistician will prepare reports as agreed upon with the SO/dual SO during the first meeting. It is required that the reports be sent by email to the NIAMS ES by the due date. The NIAMS ES will conduct a review of the reports and will follow up with the study coordinating center or statistician for any incomplete, missing data or clarification of any information provided. The NIAMS ES will distribute the reports to the SO/dual SO and the NIAMS representatives for their review.
6.2.2 Roles and Responsibilities of the SO/Dual SO
The SO/dual SO acts in an advisory capacity to the NIAMS as an independent body. The SO/dual SO is responsible for reviewing, accumulating safety data and study/trial progress to assure that the study participants are not exposed to unnecessary or unreasonable risks, and the investigator conducts the study/trial according to the protocol and with the highest scientific and ethical standards.
The SO/dual SO is paid an honorarium by the NIAMS for his/her participation in the introductory meeting, and ongoing semi-annual reviews of safety reports.
Once a study/trial is implemented, the ongoing responsibilities of the SO/dual SO are:
- Review the study protocol, DSMP, MOP, and informed consent documents, including all proposed revisions.
- Evaluate the progress of the study on an ongoing basis, as needed, including periodic assessments of data quality, participant recruitment, accrual and retention, participant risk versus benefit, performance of study site(s), and other factors that can affect the outcome.
- Evaluate safety throughout the course of the study through the routine review of aggregated AE safety data, in addition to expedited review of UPs, SAE reports, and protocol deviations impacting participant safety. The SO(s) reviews the documentation provided by the study team and makes recommendations to the NIAMS regarding protection of the study participants.
- Evaluate proposals of new sites (that differ from the approved application) and make a recommendation to the NIAMS as to whether the enrollment at the site(s) is expected to enhance overall enrollment. Activities include evaluating the patient population pool, catchment area description, recruitment plan, and target enrollment for any new clinical sites.
- Consider the impact of factors external to the study when new information, such as scientific or therapeutic developments become available and may affect safety of participants, their willingness to participate in the study or the ethics and conduct of the study.
- Assist the NIAMS by commenting on any problems with study conduct or performance.
- Ensure that the plan for maintaining the confidentiality of the study data and the results by the study team is appropriate.
- Review and evaluate requests for protocol modifications.
- Review in advance of the study initiation the study specific stopping rules and plans for interim analyses as established by the PI and selected members of the study team. These plans outline the conditions under which a study may be stopped (e.g., difficulties in recruitment, retention, obtaining outcome measures, or other issues).
- Review the interim analyses and/or accumulating data at the specified interval(s) and as appropriate and make a recommendation to continue, terminate, or modify the study based on observed benefit or harm in accordance with the planned stopping rules.
6.2.2.a Study Materials
At the beginning of a study, the SO/dual SO will review the study materials including the protocol, MOP, informed consent/assent form(s), CRFs, DSMP, and DSM report templates.
The DSMP should delineate data preparation functions, the review process, and the role of the SO/dual SO. The DSMP also specifies the content and format of the reports, their frequency, and triggers for ad-hoc reviews. Stopping rules, if appropriate, should outline the conditions under which a study may be stopped prematurely. The primary focus of the SO’s/dual SO’s monitoring activity is participant safety. The SO/dual SO may suggest modifications to the protocol, the DSMP, and the reports that will routinely be prepared by the study statistician and study team, as appropriate.
The SO/dual SO must initially review the materials prior to the commencement of recruitment to ensure they are adequate to implement the study. During the introductory meeting, the SO/dual SO will provide comments to clarify or revise parts of the study materials, if required. They will make a recommendation regarding the adequacy of the study materials prior to the start of the study.
As the study progresses, it is expected that there may be unanticipated changes to the study protocol that require updates to the study materials. Any changes to the study potentially impacting the budget must first be discussed with the NIAMS program staff. Amended materials include but are not limited to changes to the protocol, MOP, DSMP, informed consent/assent form(s), and CRFs. All changes made to the study should be reviewed by the SO/dual SO, the NIAMS, and the IRB prior to implementing the changes.
6.2.2.b DSM Reports
At predetermined intervals (typically semi-annually), the study team will prepare safety reports to be reviewed by the SO/dual SO. If the NIAMS DSM Report templates are used, they must be customized to each study and the SO/dual SO may request additional reports, as necessary. View the NIAMS DSM Report Templates for single-site open session, single-site closed session, multi-site open session, and multi-site closed session. The PI can create his/her safety reports, however, they must be reviewed and approved by the SO/dual SO.
6.2.2.c AE, SAE, UP, and Protocol Deviation Definitions
The PI and study team are expected to provide a definition of AEs, SAEs, UPs, and protocol deviation. The PI and study team may draw from OHRP guidance (https://www.hhs.gov/ohrp/regulations-and-policy/guidance/reviewing-unanticipated-problems/); for some studies, the ICH E6 definition may be more appropriate.
Define the events placing the participant at increased risk of harm or compromise the integrity of the safety data for all protocol deviations.
6.2.2.d AE Reporting
In the case where the intervention is an FDA regulated drug, device or biologic, it should include the FDA definition, grading scale and “study relatedness” criteria of AEs. All non-serious AEs (regardless of expectedness or relatedness) are reported to the DSMB/OSMB and the NIAMS (through the NIAMS ES) semi-annually or as determined by the NIAMS.
6.2.2.e Expedited Reporting of SAEs, UPs, and Protocol Deviations
The SO/dual SO will review SAEs or other expedited reports (e.g., unanticipated problems, protocol deviations impacting the safety of the research participant), and relatedness of events to study/intervention and communicate recommendations regarding SAEs to the NIAMS through the NIAMS ES.
All SAEs must be reported to the NIAMS and the SO/dual SO (through the NIAMS ES) within 48 hours of the investigator becoming aware of the event. All AEs, both serious and non-serious, related and unrelated, must be reported at predetermined intervals to the NIAMS and the SO/dual SO (through the NIAMS ES). The SO/dual SO is responsible for reviewing the event report, including the PI’s adjudication of the event. The SO/dual SO makes their own independent assessment of the event. For more information, see the NIAMS Reportable Events Requirements and Guidelines for Investigators Conducting NIAMS-Funded Clinical Research Studies.
It is the responsibility of the SO/dual SO to notify the NIAMS if a pattern of events occurs and recommend mitigation strategies (e.g., modifying the protocol to require frequent measurement of laboratory values predictive of the event). Additionally, the SO/dual SO may request individual participant records, including laboratory data, clinical records, and other study related data, to evaluate these events against the known safety profile of the study intervention and the disease. The SO/dual SO may recommend actions including partial or complete unblinding, and/or modifying/placing on hold or terminating the study.
In addition to safety monitoring, the SO/dual SO will monitor study progress including but not limited to enrollment data, demographic information, retention status, and other reports prepared by the study statistician that describe study performance and progress. The SO/dual SO will review the study’s safety report and complete a checklist indicating whether study data/progress and is adequate and make a recommendation regarding safe continuation or early termination of the trial.
6.2.2.f Interim Analysis Reports
Interim analysis may be conducted either due to pre-specified stopping rules as outlined in the protocol and at predetermined intervals, or as deemed necessary by the SO/dual SO to assess safety concerns or study futility based upon accumulating data. An interim analysis may be performed for safety, efficacy and/or futility, and the reports are prepared by the unmasked study statistician or data coordinating center responsible for generating such reports. Interim analysis reports are presented to the SO/dual SO in an unmasked format, without the masked study personnel in attendance.
- Interim analysis for safety: The SO/dual SO may review unmasked interim safety data and recommend termination or modification of a trial due to a trend toward showing harm to the research participants.
- Interim analysis for efficacy: The SO/dual SO may review unmasked interim efficacy data and recommend termination of the trial, based on the interim data, where there is overwhelming evidence that the intervention is highly beneficial.
- Interim analysis for futility: The SO/dual SO may review unmasked interim efficacy data and recommend termination of the trial with the likelihood that the treatment effect being sought, based on the interim data, is very unlikely to be established even if fully enrolled.
6.2.3 SO/Dual SO Charter
The purpose of the Charter is to outline the charge to the SO/dual SO regarding their responsibilities and processes for a NIAMS-funded study. It also outlines the responsibilities of the NIAMS and the NIAMS ES. It is intended to be a living document to be modified at any time if any processes or procedures were to change. The Charter is created by the NIAMS for individual studies that require NIAMS-appointed DSM oversight. The NIAMS ES helps facilitate modifications to the Charter.
A formal review by the SO/dual SO of the Charter takes place at the initial meeting and a re-review occurs as needed. The SO/dual SO may request modifications to the Charter to align with any specific charge he/she has to the study. The SO Charter and dual SO Charter are available on the NIAMS website.
6.2.3.a Confidentiality
Confidentiality must be maintained throughout all phases of the trial, including monitoring, preparation of interim results, review, and response to monitoring recommendations. Thus, the SO/dual SO should not receive patient identifiers, will maintain study confidentiality, and will not share data. The SO/dual SO signs a confidentiality form attesting to adhering to confidentiality. This process is completed annually by the SO/dual SO.
6.3 Internally-appointed Monitoring
For some interventional and non-interventional studies with low risk, the NIAMS may determine that the DSMP proposed by the PI, typically described in the grant application, is sufficient to ensure participant safety. In such cases, the NIAMS allows the PI to appoint data and safety monitoring oversight for the study. In general, this type of monitoring only applies to low risk studies when the PI has demonstrated in the application that he/she can establish a DSM body, often at the institution. There may also be cases where the PI proposes in the grant application that they will be responsible for conducting the monitoring for the study themselves; however, the NIAMS may determine that separate internally-appointed monitoring is required with individuals independent from the study. The NIAMS makes the final determination in the level of oversight and monitoring required for a study.
For studies where the PI selects, assembles, and manages the data and safety monitoring oversight process, the NIAMS refers to this as “internally-appointed monitoring” (rather than NIAMS-appointed monitoring, where the DSM body is appointed and managed by the NIAMS). For more information about NIAMS-appointed monitoring bodies, see sections 2.3.1, 2.3.2, and 2.3.3. The oversight and management processes for internally-appointed monitoring vs NIAMS-appointed monitoring (or externally monitored) are different.
Internally-appointed monitoring allows minimal involvement in the management and oversight processes by the NIAMS. However, there are still certain requirements for oversight and reporting data to the NIAMS that must be followed by the PI. The NIAMS must be kept informed of ongoing study progress, safety events and decisions made by the PI’s DSM body on a routine basis. This process ensures the NIAMS is meeting its obligation for data and safety monitoring oversight for the studies it supports. The NIAMS’ determination for an internally-appointed DSM body includes factors including but not limited to, number of sites, risk to human subjects, number of participants, phase of study, study population, study complexity, type of intervention, and adequacy of the DSMP reviewed with the grant application.
A call (referred to as the initial PI call) will be set-up with the PI and any member(s) of the study team who is assisting the PI, the NIAMS, and the NIAMS ES to review the NIH/NIAMS clinical trial requirements and to answer any questions on how to carry out the data and safety monitoring process.
Please note that all information shared with the NIAMS and the NIAMS ES related to data and safety monitoring oversight will be kept confidential.
6.3.1 Roles and Responsibilities of the PI
If the NIAMS determines internally-appointed monitoring is appropriate for the study, it allows the PI to take responsibility for establishing the DSM body. The PI may have decided that a DSM body similar to a DSMB/OSMB or SO/dual SO described in these guidelines (see sections 2.3.1, 2.3.2, and 2.3.3) is required to provide safety monitoring, and the NIAMS will communicate with the PI to let him/her know that it is in agreement with the DSM body proposed.
Study materials are prepared by the PI and the study team. Please refer to section 6.1.3.a for further details.
The NIAMS requires that the DSM body selected by the PI must be independent of the study and members may not be in the same division as the PI and/or co-investigators. The NIAMS does not approve the DSM body members but does screen for conflict at the start of the study. The PI is responsible for vetting the DSM body members through the NIAMS via the NIAMS ES to ensure they are free of any conflicts. The PI and the study team should provide the name, affiliation, and curriculum vitae (if available) of the proposed DSM body member(s) to the NIAMS ES for a conflict of interest check to be conducted. If any member is found in conflict, the PI must propose others for vetting through the NIAMS.
The PI must establish a process for collection, assessment, and reporting of AEs, SAEs, UPs, and protocol deviations to the DSM body, IRB, and FDA (if applicable), and describe this process in the DSMP. The NIAMS requires the PI to report SAEs, UPs, and protocol deviations that impact participant safety to the NIAMS through the NIAMS ES in an expeditious manner. These events must be reported to the NIAMS within 48 hours of the investigator becoming aware of the event. For more information see the NIAMS Reportable Events Requirements and Guidelines for Investigators Conducting NIAMS-Funded Clinical Research Studies. Please note that the NIAMS expects to receive a copy of the internally-appointed DSM body’s final assessment of the event when it becomes available.
The PI and study team are required to provide to the NIAMS through the NIAMS ES monthly status updates regarding the progress of study activities during the start-up phase until the first participant is enrolled. The first update is due 30 calendar days following the initial PI call with the NIAMS and should include a milestone timeline table and a projected enrollment graph. Once the first participant is enrolled, the PI and study team should provide monthly enrollment reports. The NIAMS ES will be in close contact with the PI and study team to provide guidance and ensure these timelines are met.
6.3.2 Responsibilities of Internally-appointed Monitoring Body
The internally-appointed DSM body is responsible for data and safety monitoring oversight. The DSM body will be responsible for reviewing study data and progress in a timely fashion to ensure participant safety and data quality. The following are some examples of responsibilities a typical DSM body will have (but should not be limited to these) and should be well described in the DSMP:
- Review and provide comment/request clarification on specified study documents (e.g. protocol, informed consent documents, DSMP).
- Evaluate the progress of the study on an ongoing basis, as needed, including periodic assessments of data quality, participant recruitment, accrual and retention, participant risk versus benefit, performance of study site(s), and other factors that can affect the outcome.
- Evaluate safety throughout the course of the study through the routine review of aggregated AE safety data, in addition to expedited review of UPs, SAE reports, and protocol deviations impacting participant safety.
- Consider the impact of factors external to the study when new information, such as scientific or therapeutic developments become available and may affect safety of participants, their willingness to participate in the study or the ethics and conduct of the study.
- Review the interim analyses and/or accumulating data at the specified interval(s) and as appropriate and make a recommendation to continue, terminate, or modify the study based on observed benefit or harm in accordance with the planned stopping rules.
- Provide approval to start, continue, or stop the study.
The PI may assign other responsibilities to the DSM body not mentioned above, and those should be documented in the DSMP.
6.3.2.a Internal Safety Monitoring Process
The PI is expected to prepare a study protocol, a MOP, informed consent/assent form(s), CRFs, a final DSMP, and DSM Report template(s) that will be used to report data to the DSM body. The NIAMS has helpful templates available that investigators can use to draft some of these documents, but it is not required that the NIAMS templates be used. The NIAMS templates can be found here: https://www.niams.nih.gov/grants-funding/conducting-clinical-research/trial-policies-guidelines-templates.
The NIAMS requests that all study materials mentioned above be submitted to the NIAMS through the NIAMS ES prior to study initiation. The NIAMS will do an administrative review of the study materials focused on quality to ensure all aspects pertaining to the data and safety monitoring oversight process are outlined clearly and consistently across all documents. Please note that this review is not focused on the scientific or methodological aspects of the study. It is acceptable for the PI to submit the materials to the NIAMS ES either before, after, or concurrently with the IRB; however, if any significant changes are requested by the NIAMS, it may require resubmission and approval of the updated materials to the IRB, or vice versa.
6.3.2.b Internal Review of Data and Safety Reports and Monitoring Body Meetings
The timeline and frequency of the review of reports and/or meetings by the internally-appointed DSM body is set by the PI and the DSM body. The NIAMS does not determine how often the internally-appointed DSM body should review data, but the PI can refer to the NIAMS-appointed DSM body guidelines as a guide (see Sections 6.1.2.c, 6.1.2.d and 6.2.2.b). Safety reports submitted to the DSM body must be shared with the NIAMS through the NIAMS ES, along with any feedback or recommendations from the DSM body immediately following the review of the reports or meeting. It is important for the NIAMS to be kept updated on the outcome of reviews conducted by the DSM body.
6.3.2.c Amended Materials
As the study progresses it is expected that there may be unanticipated changes to the study protocol that require updates to the study materials. Any changes to the study potentially impacting the budget must first be discussed with the NIAMS program staff. Amended materials include but are not limited to changes to the study’s protocol, MOP, DSMP, informed consent/assent form(s), and CRFs. All changes made to the study should be reviewed and approved by the internally-appointed DSM body and the IRB prior to implementing the changes. The NIAMS must be notified of these changes. All approved amended materials should be shared with the NIAMS through the NIAMS ES.

