April 2018
FY 2019 Congressional Justification PDF version (209 KB)
Organization Chart
View a larger version of the organization chart.
Appropriation Language
NATIONAL INSTITUTES OF HEALTH
National Institute of Arthritis and Musculoskeletal and Skin Diseases
For carrying out section 301 and title IV of the PHS Act with respect to arthritis and musculoskeletal and skin diseases, $545,494,000.
Amounts Available for Obligation
Source of Funding |
FY 2017 Final |
FY 2018 Annualized CR |
FY 2019 President's Budget |
|---|---|---|---|
| Appropriation | $557,851 | $557,851 | $545,494 |
| Mandatory Appropriation: (non-add) | |||
| Type 1 Diabetes | (0) | (0) | (0) |
| Other Mandatory financing | (0) | (0) | (0) |
| Rescission | 0 | -3,788 | 0 |
| Sequestration | 0 | 0 | 0 |
| Secretary's Transfer | -1,245 | ||
Subtotal, adjusted appropriation |
$556,606 | $554,063 | $545,494 |
| OAR HIV/AIDS Transfers | 0 | 0 | 0 |
Subtotal, adjusted budget authority |
$556,606 | $554,063 | $545,494 |
| Unobligated balance, start of year | 0 | 0 | 0 |
| Unobligated balance, end of year | 0 | 0 | 0 |
Subtotal, adjusted budget authority |
$556,606 | $554,063 | $545,494 |
| Unobligated balance lapsing | -38 | 0 | 0 |
Total obligations |
$556,568 | $554,063 | $545,494 |
1Excludes the following amounts (in thousand) for reimbursable activities carried out by this account:
FY 2017 — $3,164 FY 2018 — $3,006 FY 2019 — $2,946
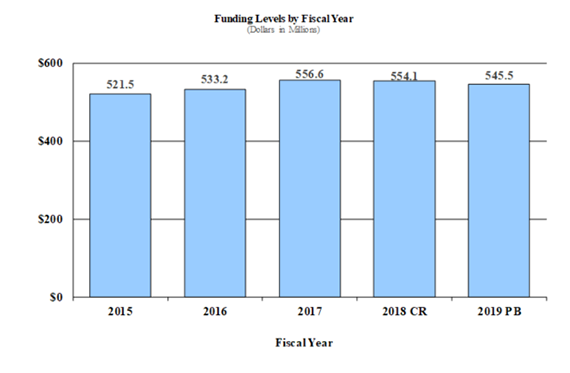
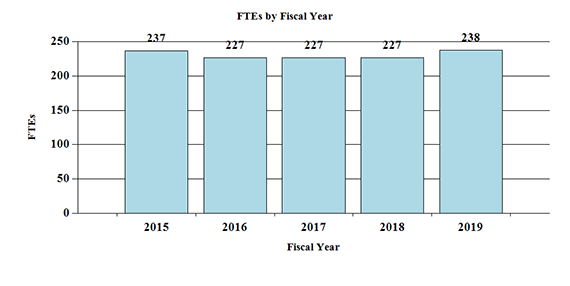
Fiscal Year 2019 Budget Graphs
History of Budget Authority and FTEs:
Authorizing Legislation
| PHS Act/ Other Citation |
U.S. Code Citation |
2018 Amount Authorized |
FY 2018 Annualized CR |
2019 Amount Authorized |
FY 2019 President's Budget |
|
|---|---|---|---|---|---|---|
| Research and Investigation | Section 301 | 42§241 | Indefinite | $554,062,634 | Indefinite | $545,494,000 |
| National Institute of Arthritis and Musculoskeletal and Skin Diseases |
Section 401(a) | 42§281 | Indefinite | Indefinite | ||
| Total, Budget Authority | $554,062,634 | $545,494,000 |
Appropriations History
| Fiscal Year |
Budget Estimate to Congress |
House Allowance |
Senate Allowance |
Appropriation |
|---|---|---|---|---|
| 2009 | $509,080,000 | $526,583,000 | $523,246,000 | $524,872,000 |
| Rescission | $0 | |||
| 2010 | $530,825,000 | $543,621,000 | $533,831,000 | $539,082,000 |
| Rescission | $0 | |||
| 2011 | $555,715,000 | $554,846,000 | $539,082,000 | |
| Rescission | $4,733,461 | |||
| 2012 | $547,891,000 | $547,891,000 | $528,332,000 | $536,801,000 |
| Rescission | $1,014,454 | |||
| 2013 | $535,610,000 | $537,233,000 | $535,786,446 | |
| Rescission | $1,071,573 | |||
| Sequestration | ($26,892,795) | |||
| 2014 | $540,993,000 | $537,398,000 | $520,053,000 | |
| Rescission | $0 | |||
| 2015 | $520,189,000 | $521,665,000 | ||
| Rescission | $0 | |||
| 2016 | $533,232,000 | $528,137,000 | $544,274,000 | $542,141,000 |
| Rescission | $0 | |||
| 20171 | $541,662,000 | $555,181,000 | $564,131,000 | $557,851,000 |
| Rescission | $0 | |||
| 2018 | $417,898,000 | $566,515,000 | $576,178,000 | $557,851,000 |
| Rescission | $3,788,367 | |||
| 2019 | $545,494,000 |
1 Budget Estimate to Congress includes mandatory financing.
Justification of Budget Request
National Institute of Arthritis and Musculoskeletal and Skin Diseases
Authorizing Legislation: Section 301 and title IV of the Public Health Service Act, as amended.
Budget Authority (BA):
| FY 2017 Actual | FY 2018 Annualized CR |
FY 2019 President’s Budget |
FY 2019 +/- FY 2018 |
|
|---|---|---|---|---|
| BA | $556,606,000 | $554,062,634 | $545,494,000 | -$8,568,634 |
| FTE | 227 | 227 | 238 | +11 |
Program funds are allocated as follows: Competitive Grants/Cooperative Agreements; Contracts; Direct Federal/Intramural and Other.
Director’s Overview
The National Institute of Arthritis and Musculoskeletal and Skin Diseases (NIAMS) is the primary Federal agency responsible for supporting medical research on diseases of the bones, joints, muscles, and skin. The diseases and conditions within the NIAMS’ mission areas touch the lives of virtually every American, impose significant financial burden, and reduce quality of life. Recent analyses from the Centers for Disease Control and Prevention (CDC) noted that arthritis is common, disabling, and a growing major health concern. More than 54 million American adults have some form of doctor-diagnosed arthritis, such as osteoarthritis, rheumatoid arthritis, gout, and fibromyalgia, and 42.8 percent of those individuals experience activity limitations as a result of their disease.1 Arthritis accounted for approximately $300 billion in economic burden in 2013, nearly one percent of the gross domestic product that year, and individuals with arthritis earned approximately nine percent less in wages than those who did not.2 Often certain groups are disproportionately affected by diseases within the NIAMS’ portfolio. For example, recently completed population-based studies of lupus, an autoimmune disease where the body’s immune system attacks healthy tissues, confirmed that women have a higher incidence of lupus than do men. The studies also revealed that the prevalence of lupus is greater in Hispanic and Asian Americans than in Whites, but that African Americans experience the highest prevalence.3,4 NIAMS is working to enhance health, lengthen life, and reduce illness and disability from these and other diseases of the bones, joints, muscles, and skin. The Institute supports novel partnerships to facilitate new avenues of research, basic science studies to understand the underlying cause of disease, and translational and clinical research to improve outcomes for patients. NIAMS is also investing in the future through innovative programs designed to retain talented young scientists and clinicians in research careers.
In 2014, NIH embarked on a partnership with pharmaceutical companies and nonprofit organizations, the Accelerating Medicines Partnership (AMP), to develop new ways of identifying and validating promising biological targets for diagnostics and drug development.
- Vital Signs: Prevalence of Doctor-Diagnosed Arthritis and Arthritis-Attributable Activity Limitation
- Medical Expenditures and Earnings Losses Among US Adults With Arthritis in 2013
- The Incidence and Prevalence of Systemic Lupus Erythematosus in San Francisco County, California: The California Lupus Surveillance Project
- The Incidence and Prevalence of Systemic Lupus Erythematosus in New York County (Manhattan), New York: The Manhattan Lupus Surveillance Program
NIAMS and the National Institute of Allergy and Infectious Diseases (NIAID) are leading projects on the autoimmune conditions rheumatoid arthritis (RA) and systemic lupus erythematosus (SLE) through the AMP RA/SLE Program. One of the most exciting results of this effort is that for the first time, scientists can study the changes taking place in the affected tissues of patients at the single-cell level. This breakthrough represents a sea change in how autoimmune diseases are studied and was accomplished through the combined resources and talents of government, industry, non-profits, and academia. The synergy from this unique partnership is facilitating advances beyond those that any one of these partners could have done alone. Thus far, the program has developed standard protocols for obtaining and analyzing patient tissues and identified and validated new research tools that enable scientists to explore disease mechanisms and follow patients clinically in ways that were previously impossible. Data generated by AMP researchers is not patentable and is made freely available to the entire research community. In January 2018, AMP researchers began releasing data and results through phase 1 of the program, which compared samples from RA and SLE patients and unaffected individuals. While phase 2 of the project, to stratify patients within their disease, is ongoing, the available data resource can be leveraged by other non-AMP scientists in academic, private, and non-profit sectors to conduct their own novel studies.
Basic research is a key focus for the NIAMS. By examining the underlying biology of living systems, both in health and in disease, prevention and treatment approaches may be revealed. For example, the human body lives with a host of microorganisms, called the microbiome. It has long been understood that disease can arise when harmful microorganisms invade. Within the last decade, researchers have begun to uncover exactly what makes up a healthy microbiome, and how it may interact with an individual’s genome and other environmental exposures to cause, or protect against, disease. Atopic dermatitis, or eczema, is a common inflammatory skin disease characterized by dry patches of skin, intense itching, redness and swelling. Earlier research by NIH and others showed that individuals with eczema have a different complement of skin bacteria than those who do not. Recently, NIAMS intramural researchers expanded on that work to identify that higher levels of one particular bacteria, Staphylococcus aureus which causes staph infections, on the skin leads to development and progression of eczema. Other researchers funded by NIAMS have translated this basic understanding to develop a new therapeutic intervention, a probiotic lotion, designed to help reduce the abundance of the harmful bacteria and restore the skin microbiome to a normal state. The microbiomes of the gut and oral cavity have also been shown to be important factors in health or disease development. Using mice that model the effects of menopause in women, NIAMS-supported researchers showed that a probiotic supplement containing a specific bacterium found in some yogurt and cheese products was able to protect against bone-loss and reduce signs of inflammation. In other work, NIAMS-supported extramural and intramural researchers identified that a certain gene variant linked to a form of arthritis called ankylosing spondylitis changes the gut microbiome in animal models, and in turn induce changes in the immune system leading to inflammation and damage to joints.
NIAMS is building upon the foundation of basic research advances and translating them into novel interventions to improve health. Epidermolysis bullosa (EB) is a skin disease that causes extensive, painful blisters and skin wounds. Prior research identified the cause of a severe, inherited form of the disease, called recessive dystrophic EB (RDEB), as a faulty gene for a protein called type VII collagen. Recently, researchers conducted a phase 1 clinical trial to test a new treatment approach to aid in healing the skin of RDEB patients. In the trial, a skin biopsy was taken from RDEB patients, cultured in the lab, and then genetically modified using a harmless virus to express a normal type VII collagen. These genetically modified cells were then grown into a sheet the size of a playing card and grafted back onto the patient’s wounds to help speed healing. Three months later, 90 percent of the transferred grafts showed the type VII collagen, and a year later 50 percent of the grafts were still intact. Investigators will continue to monitor the long-term outcomes of these patients’ grafts and are building on these promising results in a phase 2 clinical trial that will enroll more patients and examine additional outcomes, such as wound pain and itch.
In order to continue making critical progress to reduce illness and disability, a robust pipeline of talented and dedicated scientists and clinicians must be cultivated. NIAMS has implemented programs to foster the development of young clinician scientists, through both in-person forums as well as tailored small grant programs and aid their transition to research independence. The Supplements to Advance Research (STAR) award program, which began in FY 2015, supports NIAMS investigators who recently renewed their first major independent award as they work to expand their research from a single, structured project into a broader, multi-faceted research program. Reflecting NIAMS’ commitment to early-career investigators, the STAR program aligns with the trans-NIH Next Generation Researchers Initiative.
Program Descriptions and Accomplishments
Arthritis and Rheumatic Diseases: This program advances high-quality basic, translational, and clinical biomedical and biopsychosocial research to treat, cure, and prevent arthritis and autoimmune diseases. It supports the discovery and application of genetics, genomics, proteomics, immunology, and imaging insights to explain how the immune system interacts with various tissues in normal and pathological conditions. This, in turn, ensures a continuous supply of new targets on which therapies can be based. One such recent project revealed that a group of immune cells called follicular helper T cells consist of several subtypes, each of which has a distinct role in fine-tuning the immune response. While additional basic research is needed to understand how these cells regulate other parts of the immune system, the observations open up possibilities for new treatments of autoimmune diseases that are associated with overly active follicular helper T cells, and for improved vaccines that promote immune responses to protect against disease.
At the clinical end of the research continuum, NIAMS-funded investigators are conducting an open label phase II clinical trial of a drug that might enable women, whose autoimmune disease places them at increased risk of severe pregnancy complications, to deliver healthy, full-term babies. This work builds on earlier studies that defined factors that correlate with greatest risk (i.e., those women who produce an antibody known as lupus anticoagulant) and that identified the causes of the complications and an approach to prevent them (i.e., a poor blood supply to the fetus, which can be restored in mice by blocking a molecule called TNF-alpha). Other ongoing clinical projects managed by the Arthritis and Rheumatic Diseases program include several of the studies mentioned in the Patient-Reported Outcomes program portrait, and the Accelerating Medicines Partnership RA/SLE program described above.
Portrait of a Program: Patient Reported Outcomes
There are many ways to clinically measure a patient’s health status, such as blood tests and x-rays. However, these may not capture features that are key to a patient’s daily quality of life, such as pain and fatigue. To address this issue, researchers constructed PROMIS® (Patient-Reported Outcomes Measurement Information System) to assess physical, mental, and social health. This resource was originally developed for adults as an NIH Common Fund initiative administered by NIAMS and is now supported by a trans-NIH cooperative agreement.
PROMIS consists of tools, such as questionnaires, that have been rigorously developed and validated. PROMIS empowers patients with the ability to self-report measures that evaluate and monitor their pain, fatigue, physical functioning, emotional distress, and social role participation. The tools can be used to assess symptoms and functions across lifespan, disease state, and demographic groups. Today, this toolkit is used widely by researchers, is integrated into clinical trials as outcome measures, and is used by physicians in clinical practice, for example, to help guide discussions about the expected benefits of surgery.
Currently, PROMIS measures are being expanded for use in children by the Validation of Pediatric Patient-Reported Outcomes in Chronic Diseases (PEPR) Consortium. As part of this effort, NIAMS administers four cooperative agreements to investigators who are testing tools for measuring the physical, mental, and social well-being of children with a wide range of chronic conditions, including Crohn’s disease, chronic kidney disease, sickle cell disease, asthma, type 1 diabetes, atopic dermatitis, juvenile idiopathic arthritis, lupus, cancer, and inflammatory bowel disease. The PEPR tools are informing and supporting the broader NIH Environmental Influences on Child Health Outcomes (ECHO) program. PEPR investigators are working to advance several pediatric patient-reported outcomes through the FDA qualification process, which would allow their use in clinical trials to determine the efficacy of new therapies.
These patient-report tools can be used across various diseases, languages, literacy levels, and ethnic groups. They can be completed in a variety of settings, including by phone or online. PROMIS is serving as a model for other groups, including the military, to develop self-reported tools that enable patients to document issues such as sleep disturbance and pain.
Musculoskeletal Biology and Diseases: This program focuses on understanding the fundamental biology of tissues that constitute the musculoskeletal system and on translating this knowledge to a variety of diseases and conditions. The portfolio covers research into causes and treatments for chronic back and neck pain, prevention and repair strategies for joint injuries or joint diseases, and the development and application of imaging tools for assessing bone quality or monitoring osteoarthritis progression. (See program portrait for an example of how long-term public-private partnerships by NIAMS are leading to the discovery of biomarkers for disease onset and progression.) Some NIAMS-funded clinical studies are addressing controversies regarding the effectiveness of common treatments for chronic musculoskeletal disorders, such as the repeated use of intra-articular steroid injections for knee osteoarthritis. This treatment is frequently prescribed for short-term pain relief, but new data show that repeated injections every 12 weeks for two years have no long-term benefit and may be associated with accelerated cartilage loss.
Other investigators are exploring tissue engineering and regenerative medicine strategies that can be incorporated into orthopaedic procedures. For example, investigators are working to develop off-the-shelf bone products that mimic or surpass the function of natural bone grafts and are readily available for spinal fusion surgeries. In one approach tested in rats, a gelatin sponge that was seeded with bone marrow or bone-forming stem cells was implanted and shown to be comparable with currently used synthetic or natural bone grafts. In other work, researchers are exploring ways to repair large regions of bone. One set of experiments in zebrafish recently revealed some of the signals that allow missing bone to regrow into its original structure. This work is an early step toward developing a comprehensive understanding of the processes to regenerate badly damaged bone. Such knowledge may one day be translated into clinical strategies to stimulate the body’s own signaling system to repair large bone defects in a way that current medical technologies cannot achieve.
Bone Biology and Diseases: This program supports a variety of projects ranging from fundamental research into the genetic and cellular mechanisms involved in the build-up and breakdown of bone in health and disease, to epidemiologic studies of lifestyle factors that can preserve bone health. Research teams are developing improved approaches to identify people who are at increased risk of fracture (see the Bone Quality Initiative example in the program portrait below) or to treat patients who have a bone disease. While osteoporosis is the most common disease in this portfolio, investigators also are exploring the molecular basis of rare bone diseases and using this information to develop potential treatments. In 2017, for example, NIAMS-funded researchers applied powerful computing technologies to results from basic bone biology studies and identified molecules that might be turned into drugs for patients who have genetic forms of rickets.
Over the past decade, bone researchers have made fundamental discoveries related to how the skeleton influences other organ systems. One group recently discovered that bone cells produce a protein that activates certain brain cells known to suppress appetite and regulate body weight and energy metabolism. While the study was completed using mice, further evidence of the biological importance of this protein comes from people with type 2 diabetes; a higher circulating level of the protein is associated with lower weight and better blood sugar control.
Looking to FY 2019, NIAMS is partnering with the National Institute on Aging and the NIH Office of Disease Prevention’s Pathways to Prevention Program on a workshop that will clarify major questions related to the safe, long-term use of bone-building or bone-preserving drugs and will identify evidence gaps and areas of future research. Drugs such as bisphosphonates prevent fractures in older people who have osteoporosis. However, many people are reluctant to use them because of concerns about rare but serious side effects. Some experts consider the increase in under-diagnosis and under-treatment of osteoporosis to be the harbinger of a public health crisis.
Portrait of a Program: Public-Private Partnerships to Advance Biomarker Discovery
Public-private partnerships are instrumental to improving public health through biomedical research. They leverage the thinking of diverse collaborators around a common mission to create, adopt, and implement new research models that uncover causes and cures for the health challenges facing our nation. The Biomarkers Consortium is a key public-private biomedical research partnership managed by the Foundation for the National Institutes of Health. Its overall goal is to discover, develop, and seek regulatory approval for biological markers (biomarkers) to support new drug development, preventive medicine, and medical diagnostics.
A biomarker is a characteristic that can be measured as a sign of a normal or abnormal process, or of a condition or disease, and can be used to see how well a person responds to a treatment. They are commonly determined by analyzing body fluids (such as blood test or urine sample), tissues, or structures in the body (such as an X-ray). The Biomarkers Consortium projects aim to develop promising biomarkers to help accelerate the delivery of successful new technologies, medicines, and therapies for prevention, early detection, diagnosis, and treatment of disease.
NIAMS plays a major role in two key projects seeking to develop measurable biomarkers of disease progression that can facilitate drug development. One important disease is osteoarthritis (OA), the most common type of arthritis, affecting over 30 million U.S. adults. OA damages the tissue that covers the ends of bones in a joint, and allows the bones to rub together, causing pain, swelling, and loss of motion. Currently, no single test can diagnose OA. The Osteoarthritis Initiative is a public-private partnership begun more than a decade ago to gather and catalog longitudinal magnetic resonance imaging (MRI), blood, and urine data from a cohort of nearly 5,000 people with OA and healthy individuals. Researchers are analyzing the dataset to identify biomarkers of OA progression that may better diagnose and predict treatment response. They found that changes in the joint detected with MRI correlate with the amount of pain that patients experience. Scientists have also identified biochemical biomarkers of cartilage structure, collagen degradation, and bone resorption as predictors of OA progression and symptomatic pain. These new biomarkers may reveal molecular pathways that could serve as targets for future therapies.
In the Bone Quality Project, another Biomarkers Consortium partnership, investigators are leveraging data from existing studies to assess the validity of specific imaging and biochemical markers for bone health. Scientists are evaluating bone marker density and imaging-derived measurements to estimate bone strength and fracture risk. In addition, bone turnover markers are also being sought, which ultimately could be used to inform osteoporosis drug development and for patient management in clinical practice.
Muscle Biology and Diseases: This program’s overarching objective is to explain muscle’s role in health and, ultimately, to treat or prevent skeletal muscle diseases and disorders such as muscle ion channel diseases, inflammatory myopathies, disuse atrophy, skeletal muscle injury, and loss of muscle mass and strength associated with aging and diseases. For example, investigators are studying the molecules that control how much calcium is available to regulate muscle contractions. Using mice, they determined that calcium accumulation inside muscle cells causes damage that mimics what is seen in people who have a disease called malignant hyperthermia. They then successfully treated the animals with a drug that is FDA-approved for conditions that are associated with similar cell stress responses.
Pompe disease is an inherited condition where individuals lack an enzyme to metabolize the sugar glycogen, and as a result, glycogen builds up in the muscles. If not detected early in babies with the condition, their muscles do not develop properly. Without treatment to replace the missing enzyme, heart and breathing problems and even death can occur. After decades of work, a group of investigators developed a gene-transfer approach that, when recently tested in mice, shows promise for treating this rare condition. While the study’s main goal was to determine if immune reactions to the current enzyme replacement therapy given to patients could be prevented, it also demonstrated that the technique could potentially replace the existing treatment. These results directly contributed to an FDA-approved clinical trial that NIAMS began funding in FY 2017.
While the examples mentioned above may lead to new treatments, other NIAMS-funded studies may lead to improvements in how existing therapies are prescribed. Researchers examined cell culture and mouse models to understand how glucocorticoids preserve muscle function in boys who have Duchenne muscular dystrophy. They found that weekly dosing increases the level of two genes involved in muscle cell membrane repair, while daily dosing activates cell pathways that cause muscle to shrink and weaken. If the responses also occur in patients, this study could directly inform prescription instructions that would maximize the drugs’ therapeutic benefit while minimizing negative side-effects. This work could also extend beyond the muscular dystrophies, as approximately one percent of the entire U.S. population is treated chronically with glucocorticoids for other conditions.
Skin Biology and Diseases: This program’s support for basic, translational, and clinical research includes work on the developmental and molecular biology of skin, the skin as an immune organ, and the genetics of skin diseases. Investigators studying the causes of ectodermal dysplasias – a family of disorders where skin, hair, nails, sweat glands, taste buds, and teeth develop or function incorrectly – recently identified some of the molecules that are involved in the condition’s more debilitating symptoms. This linkage of patients’ genetic defects to specific disease manifestations suggests that known compounds which alter the pathways involved may be an effective treatment for preventing or healing the overgrown, painful cracks that develop on patients’ palms and soles.
Wound healing is another key component of this portfolio. NIAMS-funded investigators recently discovered that cells involved in recovery from deep cuts—which often involves scaring—can also become fat cells that underlie healthy skin tissue. Although little is known about how to direct skin repair toward regenerating normal skin instead of scaring, hair follicles may hold a clue because they seem to be a pre-requisite for the formation of these new fat cells. This and related findings are opening new avenues of research which could lead to novel treatment strategies for severe scaring and fibrotic diseases.
Itch, like wound healing, is a common condition that falls under this program. Scratching in response to an itch is a contagious behavior in mice as well as in people. Recent research into the brain changes that occur when one mouse sees another scratching has uncovered a neurotransmitter that is responsible for contagious itch. Although it is too early to tell what the immediate impact of the findings will be, the discovery opens the possibility of novel interventions to treat chronic itch that target the brain directly.
Intramural Research Program (IRP): NIAMS’ IRP conducts innovative basic, translational, and clinical research relevant to the NIAMS mission and trains investigators who are interested in related careers. Its basic and physician scientists study the genetics, etiology, pathogenesis, and treatment of rheumatic, autoimmune, inflammatory, bone, skin, and muscle diseases. Foundational research by NIAMS intramural scientists on immune cells and how they communicate with each other led to the discovery of the JAK-STAT signaling pathway and formed the scientific basis for development of a new class of drugs, called Jakinibs. Some Jakinibs have been FDA-approved for the treatment of RA and certain cancers. Recently, NIAMS intramural investigators examined if one of these drugs – tofacitinib, which is approved for treatment of RA – could alleviate disease symptoms in a mouse model of lupus. Based on positive findings in mice, NIAMS clinicians have initiated a phase 1b clinical trial to determine the safety and tolerability of tofacitinib for SLE patients with active disease. The program portrait below highlights additional work being pursued in the NIAMS IRP to understand how autoimmune diseases develop.
In FY 2018, the Dermatology Branch joined the NIAMS IRP, transitioning from the National Cancer Institute intramural program. This addition provides a great opportunity for synergy across the scientific programs within NIAMS, as well as between NIAMS and many others across the NIH. The Dermatology Branch conducts basic and clinical investigations of skin biology and researches the causes, diagnosis, and treatment of skin disease. Research interests include the development and maintenance of normal skin and changes that occur in skin cancer; understanding interactions between skin and the microbiome; and immune cell function in the skin. In addition, the Branch supports the Dermatology Consultation Service in the NIH Clinical Center, one of the busiest clinical services at the research hospital and is responsible for all outpatient and inpatient dermatologic patient care.
Portrait of a Program: NIAMS Intramural Research to Understand the Mechanisms of Autoimmune Disease
Autoimmune diseases occur when a person’s immune system, which normally acts to fight infection, erroneously recognizes healthy tissues of the body as foreign and begins to attack them. Over decades, a scientific consensus has emerged that a combination of a genetic susceptibility plus a triggering event lead to the development of autoimmunity. NIAMS intramural scientists are exploring how certain genetic changes may predispose people to develop autoimmunity. For example, mutations to a cell signaling protein called Fas have previously been associated with autoimmunity. Recently, the NIAMS Autoimmunity Branch demonstrated in cell lines and a mouse model that normal Fas protein protects against autoimmunity, but through a different mechanism than has long been believed, revealing an additional path for future studies. NIAMS intramural researchers are also examining the genome of individuals with autoimmune disease to identify other genes that may contribute to disease progression and severity. Investigators from the NIAMS Systemic Autoimmunity Branch, in collaboration with teams from NHGRI and Turkey, identified genetic mutations that altered the C1r protein in a family with several members affected by early-onset lupus. C1r is a key enzyme in the complement system, a series of proteins that enhance the immune system’s ability to clear infections and damaged cells. The C1r gene mutation carried by this family leads to a C1r protein that does not function normally, and lower total complement activity was observed in the patients. Interestingly, while the affected brother and sister carried the same genetic anomaly, their disease severity was different. Future studies will seek to identify hormonal and genetic variants that may contribute to disease progression and severity in lupus patients carrying the C1r mutation.
NIAMS intramural researchers are also working to identify how certain environmental exposures may trigger autoimmunity. For example, levamisole, a therapeutic treatment for parasitic infections, was removed from the U.S. market in 1999 because it was associated with drug-induced autoimmunity. Recently, scientists from the NIAMS Vasculitis Translational Research Program determined that levamisole binds to specific receptors on the cell surface of a subset of white blood cells, called neutrophils, causing them to activate as they would in response to a bacterial infection. The receptors levamisole binds with were previously known to be present on nerve cells, but not on immune cells. This finding suggests the nervous system may communicate with white blood cells via neurotransmitters and could indicate a novel target for future therapeutic interventions.
In addition, IRP researchers are seeking factors that impact disease outcomes for autoimmune disease patients. For example, autoimmune myopathy is a muscle disease where patients produce antibodies that damage skeletal muscles. Prior research has shown that statin use is common among patients with a particular form of autoimmune myopathy, and that immunosuppressive treatment could improve muscle strength in these patients. Recent studies by the Muscle Diseases Unit of the NIAMS IRP revealed that younger patients, who tend to have less exposure to statins, experience more severe disease, and recover more slowly than older patients. Future studies will aim to understand why younger patients have worse outcomes and improve treatment strategies.
Research Management and Support (RMS): The RMS budget supports the scientific, administrative management, and information technology activities associated with the NIAMS’ day-to-day operations. In FY 2017, NIAMS managed more than 1,178 research grants and centers, as well as 37 research and development contracts and 298 individual and institutional full-time research training positions. NIAMS supported 550 clinical research studies, including 63 clinical trials.
NIAMS launched a redesigned website in FY 2017, in alignment with its mission of supporting information dissemination. The site, which is hosted in the cloud, is built with an open source content management system that promotes streamlined workflow processes and rapid content updating. The website can be displayed across multiple screen sizes, including mobile devices, and provides anytime-anywhere access to information and resources, including a searchable funding database, training opportunities, and health information. The website features a new Community Outreach portal that was designed to empower organizations and community leaders that reach diverse, underserved populations with health education tools. In addition, a large multi-lingual collection of information and resources is available and can be tailored to audiences at local levels. Together, these platforms provide NIAMS with a range of complementary outreach strategies to share research results and provide evidence-based health information to reduce disease burden across diverse populations.
Details of Full-Time Equivalent Employment (FTE)
| OFFICE/DIVISION | FY 2017 Final |
FY 2018 Annualized CR |
FY 2019 President's Budget |
||||||
|---|---|---|---|---|---|---|---|---|---|
| Civilian | Military | Total | Civilian | Military | Total | Civilian | Military | Total | |
| Intramural Research Program | |||||||||
| Direct: | 126 | 1 | 127 | 126 | 1 | 127 | 137 | 1 | 138 |
| Reimbursable: | 1 | - | 1 | 1 | - | 1 | 1 | - | 1 |
| Total: | 127 | 1 | 128 | 127 | 1 | 128 | 138 | 1 | 139 |
| Office of Extramural Activities | |||||||||
| Direct: | 47 | - | 47 | 47 | - | 47 | 47 | - | 47 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 47 | - | 47 | 47 | - | 47 | 47 | - | 47 |
| Office of the Director | |||||||||
| Direct: | 52 | - | 52 | 52 | - | 52 | 52 | - | 52 |
| Reimbursable: | - | - | - | - | - | - | - | - | - |
| Total: | 52 | - | 52 | 52 | - | 52 | 52 | - | 52 |
Total |
226 | 1 | 227 | 226 | 1 | 227 | 237 | 1 | 238 |
| Includes FTEs whose payroll obligations are supported by the NIH Common Fund. | |||||||||
| FTEs supported by funds from Cooperative Research and Development Agreements. | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 | 0 |
FISCAL YEAR |
Average GS Grade |
||||||||
| 2015 | 12.5 | ||||||||
| 2016 | 12.4 | ||||||||
| 2017 | 12.5 | ||||||||
| 2018 | 12.5 | ||||||||
| 2019 | 12.5 | ||||||||
Detail of Positions
| GRADE | FY 2017 Final |
FY 2018 Annualized CR |
FY 2019 President's Budget |
|---|---|---|---|
| Total, ES Positions | 1 | 1 | 1 |
| Total, ES Salary | 187,000 | 187,000 | 187,000 |
| GM/GS-15 | 21 | 21 | 21 |
| GM/GS-14 | 27 | 27 | 27 |
| GM/GS-13 | 52 | 52 | 56 |
| GS-12 | 21 | 21 | 22 |
| GS-11 | 7 | 7 | 7 |
| GS-10 | 0 | 0 | 0 |
| GS-9 | 7 | 7 | 7 |
| GS-8 | 5 | 5 | 5 |
| GS-7 | 4 | 4 | 4 |
| GS-6 | 2 | 2 | 2 |
| GS-5 | 1 | 1 | 1 |
| GS-4 | 1 | 1 | 1 |
| GS-3 | 0 | 0 | 0 |
| GS-2 | 0 | 0 | 0 |
| GS-1 | 0 | 0 | 0 |
| Subtotal | 148 | 148 | 153 |
| Grades established by Act of July 1, 1944 (42 U.S.C. 207) | 0 | 0 | 0 |
| Assistant Surgeon General | 0 | 0 | 0 |
| Director Grade | 1 | 1 | 1 |
| Senior Grade | 0 | 0 | 0 |
| Full Grade | 1 | 1 | 1 |
| Senior Assistant Grade | 1 | 1 | 1 |
| Assistant Grade | 0 | 0 | 0 |
| Subtotal | 3 | 3 | 3 |
| Ungraded | 91 | 91 | 97 |
| Total permanent positions | 148 | 148 | 153 |
| Total positions, end of year | 239 | 239 | 250 |
| Total full-time equivalent (FTE) employment, end of year |
227 | 227 | 238 |
| Average ES salary | 187,000 | 187,000 | 187,000 |
| Average GM/GS grade | 12.5 | 12.5 | 12.5 |
| Average GM/GS salary | 110,203 | 112,184 | 112,639 |
1 Includes FTEs whose payroll obligations are supported by the NIH Common Fund.

